P417
Urease is an immunogenic protein that is recognized by antibodies in human sera. Urease contains two subunits that are 21 and 65 kD in size. The protein is characterized by a highly-mobile helix-turn-helix motif. This motif, which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urease exists as an apoenzyme and its activation is dependent on the proteins that shuttle Ni ions into the cell for incorporation in the enzyme’s active site.
Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR spectroscopy allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used FTIR spectroscopy to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus.
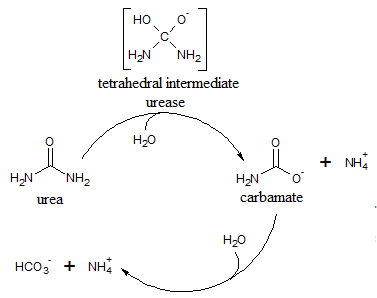
Reaction 1
.
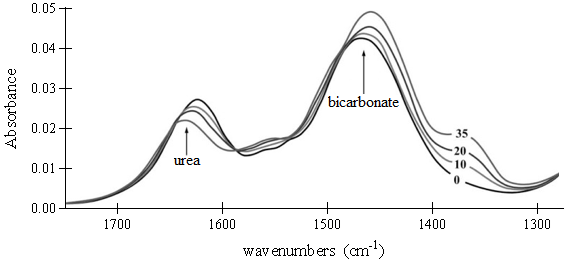
Figure 1. FTIR spectra of the substrate (urea) and product (bicarbonate; NaHCO3) in a reaction mixture containing 0.4 μg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
