P416
Asymmetric synthesis of new organic compounds can be carried out using organometallic (organic compounds which the metal atom is directly bonded to a carbon) reagents such as alkyl lithium (RLi) and alkyl magnesium (RMgX; Grignard reagent, R is any alkyl group and X is any halogen) reagents. Organometallic reagents are generated by reacting haloalkanes with either lithium or magnesium in ethereal solvents. The alkyl group in organometallic reagents (Cδ-) shows reverse polarization when compared to haloalkanes (Cδ+). These reagents provide a carbon-based nucleophile (R:–) which can attack the carbonyl group of an aldehyde or ketone to generate alcohol and a new C-C bond. Reacting an ester with RMgX results in the formation of two new C-C bonds.
The following reactions show the asymmetric synthesis of an organic compound C using RMgX.
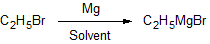
Reaction 1
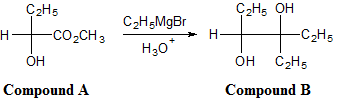
Reaction 2
The conversion of Compound A to Compound B requires 3 moles of Grignard reagent for each mole of Compound A.
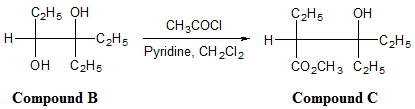
Reaction 3
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
