P650
“Because a wide array of important compounds (such as natural products, pharmaceuticals, and functional polymers/materials) contain nitrogen, the development of methods for the efficient formation of C−N bonds is a central challenge in organic chemistry. The formation of a Csp3−N bond through a substitution reaction (SN2) between a nitrogen nucleophile and an alkyl halide is a classic method that is widely used, despite its limitations.
A laboratory discovers that the use of a preliminary Copper Iodide (CuI) catalyst, in coordination with a specific wavelength of light, could successfully alkylate terminal amine groups in good yields.
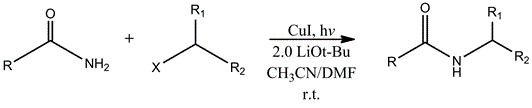
Figure 1. Generic reaction scheme for the alkylation of an amine.
Table 1. Modification of reaction conditions.
| Entry | Conditions | Yield |
| 1 | 10% CuI, 254 nm hv | 90% |
| 2 | No CuI | <2% |
| 3 | No hv | <2% |
| 4 | CuBr, instead of CuI | 82% |
| 5 | CuCl, instead of CuI | 78% |
| 6 | hv (300 nm) | 10% |
| 7 | hv (100 W Hg lamp) | <2% |
| 8 | 0 °C | 33% |
| 9 | 5.0% CuI | 87% |
| 10 | 2.5% CuI | 83% |
| 11 | 1.0% CuI | 69% |
| 12 | 0.5% CuI | 30% |
”
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
