P428
A student prepares solutions of saturated NaNO3, NaCl, and Ce2(SO4)3 • 9H2O. The student confirms the solutions are saturated by adding more solute and noticing a precipitate at the bottom of each solution. The contents of each solution are heated gently, and the precipitate in the NaNO3 solution dissolves completely. The student notices no visual change in the quantity of NaCl dissolved and notices that more precipitate forms when the Ce2(SO4)3 • 9H2O solution is heated.
.
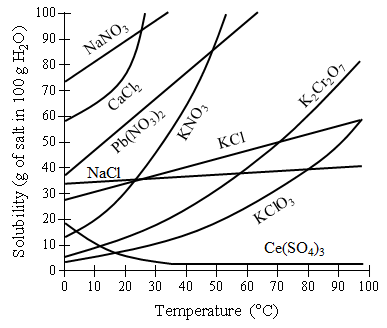
Figure 1. Solubility vs. temperature graph of various compounds.
.
.
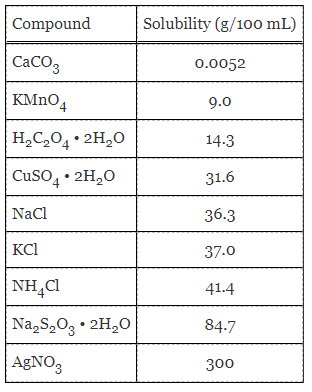
Table 1. Solubility of various compounds at 30 °C.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
