P411
The decomposition of hydrogen bromide, HBr, is a demonstration of how diverse environments can influence chemical kinetics. Under certain conditions, the decomposition proceeds in a single elementary reaction:
Reaction A
Reaction A is exothermic, with standard activation energy of 196 kJ/mol. In some cases, a metal catalyst is introduced, and in the presence of metal surfaces like gold and platinum, the activation energies are more closely approximated at 115 kJ/mol and 72 kJ/mol, respectively. Data collected from an experiment with Reaction A are provided in Table 1.
.
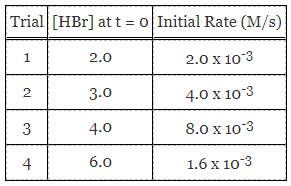
Table 1. Comparison of reaction rates at various HBr concentrations.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
