P408
Analyte extraction from a solution depends on differential solubility of compounds and is used to draw the required compound into aqueous or organic layer. The distribution coefficient (K) is the ratio of solubility of a compound in organic layer to its solubility in aqueous layer:

Equation 1
In Equation 1, x + y = total weight in grams of the compound present in the mixture. Extraction also depends on solvent immiscibility and density; solvents with lower density form the top layer when a water-organic solvent pair is used. Repeated small volume extractions are more efficient compared to single large volume extractions.
Acid-base extraction may be used to convert non-ionic organic soluble compounds to ionic water soluble forms by changing pH. Amines, carboxylic acids and phenols (here R’ is C6H5) may be purified in this way.
Amines RNH2 + H+ ↔ RNH3+Phenols R’OH + OH– ↔ R’O– + H2OCarboxylic Acids R”COOH + OH– ↔ R”COO– + H2O
These reactions along with other acid-base reactions impact the extraction ability of proteins for analysis. Researchers extracted three proteins from C. elegans, a model organism for understanding animal development. These three proteins are suspected to impact development through paracrine and autocrine mechanisms. The extraction efficiencies of the proteins varied considerably depending on the solutions used, as shown in Figure 1.
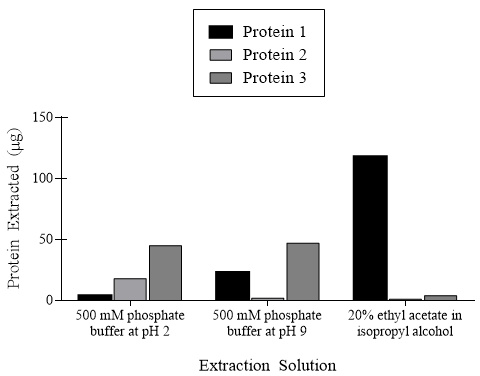
Figure 1. Quantity of three proteins extracted in different extraction solutions.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
