P404
Prenylation is the addition of nonpolar molecules to proteins or chemical compounds. Farnesylation is a specific form of prenylation that involves the addition of an isoprenyl group to a cysteine residue in a protein.
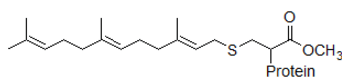
Reaction 1. Farnesyl diphosphate + protein ↔ protein-S-farnesyl + diphosphate
Biologically, this reaction is a form of post-translational modification, by which farnesylation facilitates protein-protein interactions and protein-membrane interactions. Proteins that undergo prenylation include Ras, which plays a central role in the development of cancer. This observation suggests that inhibitors of prenylation enzymes could potentially reduce the growth of tumors.
A mutation of the ras gene is present in 50% of colon cancer cases and 90% of clinically diagnosed cases of pancreatic cancer. The farnesyl-protein transferase enzyme is responsible for the farnesylation of the Ras protein in vivo. This reaction is critical for the association of the Ras protein with the plasma membrane, which leads to subsequent cell-transforming activity.
Efforts were made by researchers to identify inhibitors of the farnesyl-protein transferase enzyme. The study identified two novel and potent inhibitors of the enzyme: actinoplanic acid A and B. The structures of these two compounds are shown below. The effectiveness of each compound was measured by calculating the half maximal inhibitory concentration (IC50), the half maximal effective concentration (EC50), and the median lethal dose (LD50).
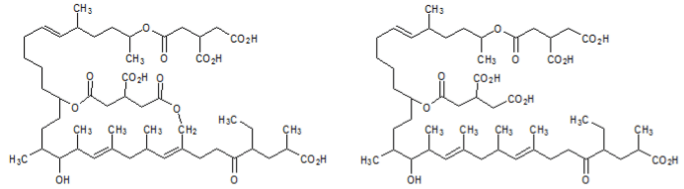
Figure 2. Actinoplanic acid A (left) and Actinoplanic acid B (right)
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
