P1002
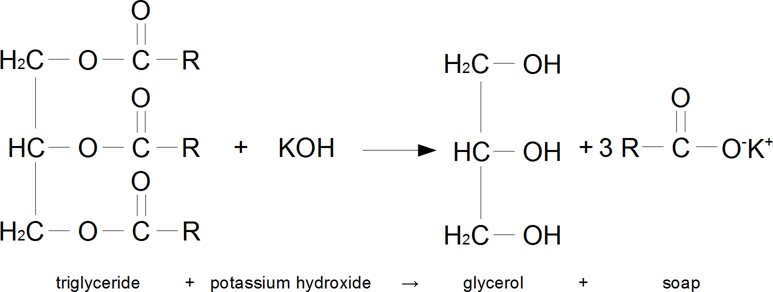
Saponification (soap making) is the chemical process of producing glycerol and carboxylate salts from the reaction of fats or oils and a base. This carboxylate salt produced is soap. Typically, the fat used is a triglyceride, and the base is sodium or potassium hydroxide. A typical metric that quantifies this chemical process is the saponification number, the number of milligrams of KOH that has to be added in order to saponify 1 g of fat (or oil).
Figure 1. Example of saponification reaction.
A chemist prepares an experiment where two tanks are filled with ethyl acetate (CH3COOC2H5) and sodium hydroxide (NaOH), respectively. These tanks have outlets, connected to pipes, that let their contents mix in a third tank (initially with a non-reactive and electrically neutral solution), where the saponification reaction occurs, creating acetate ion and ethanol.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!