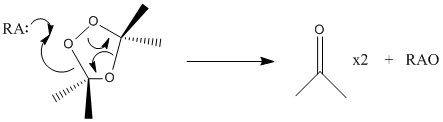P1001
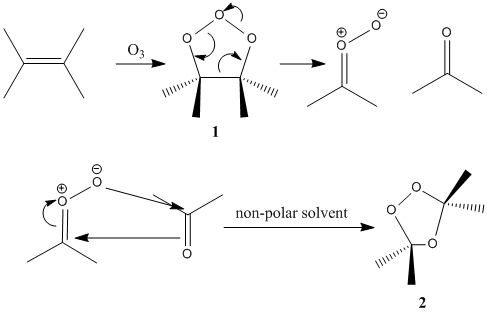
Ozone is a toxic gas which forms explosive ozonides with many olefins. Ozone readily attacks ethylenic linkages and from the products, carbonyl compounds can be obtained. The process results in separation of the carbon atom originally joined by the double bond. The identities and yields of carbonyl products provide information on the positions of the double bonds in the olefin. Hence ozonolysis is frequently used in structure determination as well as for synthetic purposes.
One laboratory discovered that the preferred mode of addition to most olefins is via a molonozide (1). The molonozide rapidly decomposes to carbonyl and peroxy products which can rapidly recombine in non-polar solvents to give an ozonide (2).
The ozonide can then be hydrolyzed using a reductive workup in order to break the peroxy link (RA = Reducing agent).
A student prepares a reaction mixture of 2,3-dimethylhex-2-ene in solution, and bubbles ozone (O3) through the mixture for 30 minutes. The student then washes the reaction contents with water into a round-bottom flask containing a reducing agent. The contents from the round-bottom are separated by steam distillation and identified using 1H-NMR and IR Spectroscopy.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!