P422
Aspirin (acetylsalicylic acid, MW: 180g/mol) is a relatively stable compound. However, exposure of aspirin to heat or moisture hastens its decomposition to acetic acid and salicylic acid, as depicted below:
.
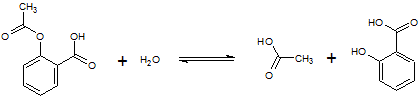
Reaction 1
.
In the first trial, the student weighs 90 mg of aspirin and dissolves it in water to make a 10 mL solution. After 5 minutes of incubation at 90 °C, the reaction was stopped upon insertion in an ice bath with Fe(NO3)3. It was later found that 0.05 mmol of salicylic acid was produced.
In a second trial, the student repeats the experiment, but with 60 mg of aspirin and finds that 0.033 mmol of salicylic acid is produced.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
