P420
A haloacid is with the formula HXO4 is purified and titrated with a strong base. The acid had a strong tendency to decompose into various gases including O2, particularly at high concentrations in the presence of base.
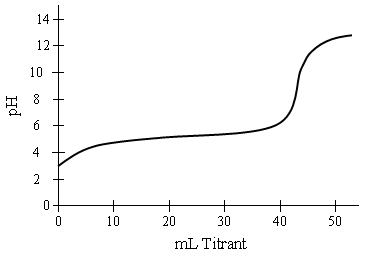
Figure 1. Titration of dilute haloacid acid with a 0.10 M strong base.
Researchers produced variants of the haloacid with other halides, nonmetals, and metalloids. They theorized that the electronegativity of the oxygen-complexed central atom, such as a halogen, would determine both the acidity and the tendency to decompose in the presence of a base.
The same effect was difficult to recreate with bases, since the conjugate acids of the strong bases tested were monoatomic. Such bases had the formula M(OH)x and their solubility varied considerably depending on the metal used.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
