P412
A chemist collected four known silver alloys and conducted a series of experiments to study the properties of each alloy. Data from the experiments were recorded in Table 1. The samples contained 0.036%, 0%, 0.004%, and 0.024%, respectively of an unknown substance.
In the first set of experiments, the chemist dissolves a 22 g mass of one of the alloys listed in Table 1 into a nitric acid solution. When NaCl is added to the solution, all the silver is precipitated. The precipitate weighs 14 g.
In the second set of experiments, Electrum is placed in a solution of sulfuric acid. An oxidation-reduction reaction occurs and zinc sulfide precipitates from the solution.
.
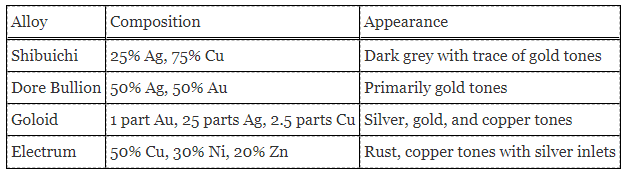
Table 1. Properties and composition of various alloys.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
