P632
“Maintaining proper acid-base balance in the body is crucial to sustaining life. Blood pH is tightly regulated between a pH of 7.35 and 7.45; deviations from these values result in acidosis or alkalosis, respectively. Carbon dioxide (CO2) provides the body’s most important buffering system and can be quickly regulated by altering respiration. CO2 is converted to HCO–3 and H+ by the reaction provided below, which is catalyzed by carbonic anhydrase:
a
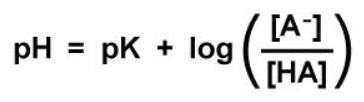
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
