P433
In chemistry, some molecules cannot be depicted by a single Lewis structure because they have two or more delocalized electrons. Instead, the most accurate way to represent such compounds is through potential alternative forms, or resonance structures, all of which contribute to the “”actual”” or true molecular arrangement. The concept, often simply described as resonance, has provided valuable insight into the chemical and physical properties of molecules, including their reactivity, stability, and the nature of conjugated systems.
An example of a compound that exhibits resonance is urea, CO(NH2)2, a metabolic waste product that is excreted by humans and other animals in urine and sweat.
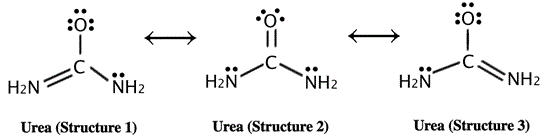
In urea, nitrogen provides its pair of non-bonding electrons to create an additional covalent bond with the carbon atom (in this case, forming a π bond). As depicted by the resonance structures, the electronegative oxygen atom of urea also withdraws the π electrons from the C=O bond. These are incorporated in a third, non-bonding orbital.
Typically, the delocalization of electrons lowers the total potential energy of a compound, and makes it more stable than any set individual structure. This is true for urea as well; while the internal resonance of the compound provides high stability, it is also responsible for the low bond energy and fact that urea is essentially of no metabolic use to mammals.
.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!
