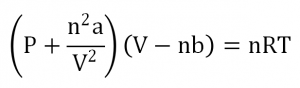P1012
The ideal gas law, PV = nRT where P = pressure, V = volume, n = moles, R = gas constant, and T = temperature, is an equation that gives the state of an ideal gas. The equation gives rise to many other gas laws, such as Boyle’s law which predicts an inverse proportionality of pressure and volume of constant temperature gas system. In reality, many gases do not behave according to the gas law. The van der Waals equation attempts to better predict the behavior of such gases and is represented as
where a is the measure of attraction between the gas particles and b is the volume excluded by one mole of particles.
Find an error? Take a screenshot, email it to us at error@mytestingsolution.com, and we’ll send you $3!